PATHPROFIL
Portable Automated System for Rapid Detection of Urinary Tract Infection Pathogens and Antibiotic Resistance
About us
PATHPROFIL is a cutting-edge research project developing an integrated portable bioanalytical system capable of rapidly identifying urinary tract infection (UTI) pathogens and their resistance to antibiotics. Combining advanced optical biosensing, microfluidic automation, and molecular diagnostics, PATHPROFIL aims to deliver results within less than two hours — compared to the 24–48 hours required for conventional urine cultures.


WHY PATHPROFIL MATTERS
Urinary tract infections are among the most common bacterial infections worldwide, affecting over 150 million people annually and accounting for a major share of outpatient antibiotic prescriptions. However, most current diagnostic tests rely on time-consuming culture-based techniques, delaying targeted treatment and often leading to empirical use of broad-spectrum antibiotics.
PATHPROFIL offers a new generation of diagnostic technology that can detect key bacterial pathogens such as E. coli, E. faecalis, K. pneumoniae, P. mirabilis, and P. aeruginosa directly from urine samples, while also identifying major antibiotic resistance genes (e.g. blaTEM-1, sul2, blaCTX-M, vanA, vanB).
How it works
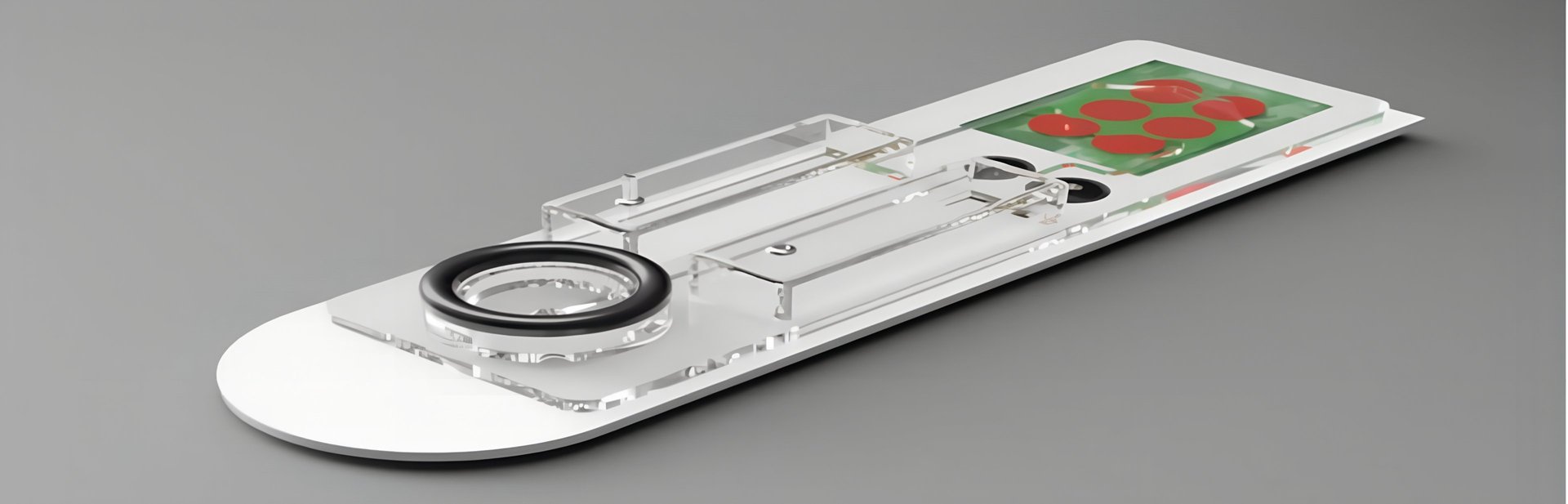
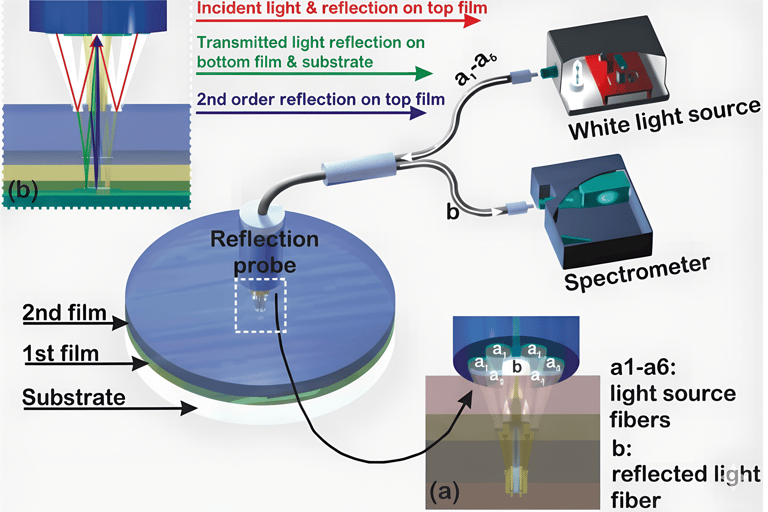
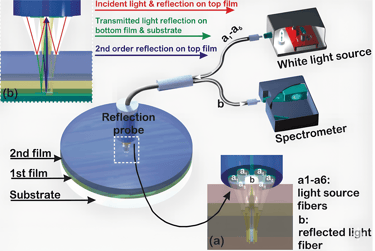
At the heart of PATHPROFIL lies White Light Reflectance Spectroscopy (WLRS) — an optical technique developed by ThetaMetrisis that measures subtle light interference changes as biological reactions occur on a silicon chip surface.
The PATHPROFIL device integrates:
a biosensor module based on WLRS for real-time detection,
a microfluidic cartridge for automated sample handling,
and an AI-supported software platform for data processing and visualization developed by QUBITEQ.
Together, these components form a compact, user-friendly instrument suitable for use in hospitals, clinics, and remote healthcare settings.
Clinical and societal impact
By reducing diagnostic time from 2 days to less than 2 hours, PATHPROFIL will:
Enable faster and more precise treatment, improving patient outcomes;
Support antibiotic stewardship by identifying resistance before prescribing;
Reduce hospital stays and healthcare costs;
Facilitate testing in resource-limited or point-of-care environments.
Clinical validation will take place at the Biomedical Engineering Laboratory of the National and Kapodistrian University of Athens (NKUA) and the Attikon University Hospital, ensuring that the PATHPROFIL system meets medical accuracy and reliability standards.


The Consortium
Medica School, National & Kapodistrian University of Athens (Coordinator) – Biomedical research and clinical validation
ThetaMetrisis S.A. – Optical biosensor design and hardware
QUBITEQ Ltd. – System integration and software development
ATG S.A. – Clinical testing and sample analysis
Hellenic Mediterranean University (ELMEPA) – Microfluidic component engineering











Get in touch
Share with visitors how they can contact you and encourage them to ask any questions they may have.
